- Information Technology
- Data Infrastructure
- Data Tools
- Desktops, Laptops and OS
- Chip Sets
- Collaboration Tools
- Desktop Systems - PCs
- Email Client
- Embedded Systems
- Hardware and Periferals
- Laptops
- Linux - Open Source
- Mac OS
- Memory Components
- Mobile Devices
- Presentation Software
- Processors
- Spreadsheets
- Thin Clients
- Upgrades and Migration
- Windows 7
- Windows Vista
- Windows XP
- Word Processing
- Workstations
- Enterprise Applications
- IT Infrastructure
- IT Management
- Networking and Communications
- Bluetooth
- DSL
- GPS
- GSM
- Industry Standard Protocols
- LAN - WAN
- Management
- Mobile - Wireless Communications
- Network
- Network Administration
- Network Design
- Network Disaster Recovery
- Network Interface Cards
- Network Operating Systems
- PBX
- RFID
- Scalability
- TCP - IP
- Telecom Hardware
- Telecom Regulation
- Telecom Services
- Telephony Architecture
- Unified Communications
- VPNs
- VoIP - IP Telephony
- Voice Mail
- WAP
- Wi-Fi (802.11)
- WiMAX (802.16)
- Wide Area Networks (WAN)
- Wireless Internet
- Wireless LAN
- Security
- Servers and Server OS
- Software and Web Development
- .Net Framework
- ASPs
- Application Development
- Application Servers
- Collaboration
- Component-Based
- Content Management
- E-Commerce - E-Business
- Enterprise Applications
- HTML
- IM
- IP Technologies
- Integration
- Internet
- Intranet
- J2EE
- Java
- Middleware
- Open Source
- Programming Languages
- Quality Assurance
- SAAS
- Service-Oriented Architecture (SOA)
- Software Engineering
- Software and Development
- Web Design
- Web Design and Development
- Web Development and Technology
- XML
- Storage
- Agriculture
- Automotive
- Career
- Construction
- Education
- Engineering
- Broadcast Engineering
- Chemical
- Civil and Environmental
- Control Engineering
- Design Engineering
- Electrical Engineering
- GIS
- General Engineering
- Industrial Engineering
- Manufacturing Engineering
- Materials Science
- Mechanical Engineering
- Medical Devices
- Photonics
- Power Engineering
- Process Engineering
- Test and Measurement
- Finance
- Food and Beverage
- Government
- Healthcare and Medical
- Human Resources
- Information Technology
- Data Infrastructure
- Data Tools
- Desktops, Laptops and OS
- Chip Sets
- Collaboration Tools
- Desktop Systems - PCs
- Email Client
- Embedded Systems
- Hardware and Periferals
- Laptops
- Linux - Open Source
- Mac OS
- Memory Components
- Mobile Devices
- Presentation Software
- Processors
- Spreadsheets
- Thin Clients
- Upgrades and Migration
- Windows 7
- Windows Vista
- Windows XP
- Word Processing
- Workstations
- Enterprise Applications
- IT Infrastructure
- IT Management
- Networking and Communications
- Bluetooth
- DSL
- GPS
- GSM
- Industry Standard Protocols
- LAN - WAN
- Management
- Mobile - Wireless Communications
- Network
- Network Administration
- Network Design
- Network Disaster Recovery
- Network Interface Cards
- Network Operating Systems
- PBX
- RFID
- Scalability
- TCP - IP
- Telecom Hardware
- Telecom Regulation
- Telecom Services
- Telephony Architecture
- Unified Communications
- VPNs
- VoIP - IP Telephony
- Voice Mail
- WAP
- Wi-Fi (802.11)
- WiMAX (802.16)
- Wide Area Networks (WAN)
- Wireless Internet
- Wireless LAN
- Security
- Servers and Server OS
- Software and Web Development
- .Net Framework
- ASPs
- Application Development
- Application Servers
- Collaboration
- Component-Based
- Content Management
- E-Commerce - E-Business
- Enterprise Applications
- HTML
- IM
- IP Technologies
- Integration
- Internet
- Intranet
- J2EE
- Java
- Middleware
- Open Source
- Programming Languages
- Quality Assurance
- SAAS
- Service-Oriented Architecture (SOA)
- Software Engineering
- Software and Development
- Web Design
- Web Design and Development
- Web Development and Technology
- XML
- Storage
- Life Sciences
- Lifestyle
- Management
- Manufacturing
- Marketing
- Meetings and Travel
- Multimedia
- Operations
- Retail
- Sales
- Trade/Professional Services
- Utility and Energy
- View All Topics
Share Your Content with Us
on TradePub.com for readers like you. LEARN MORE
Request Your Free White Paper Now:
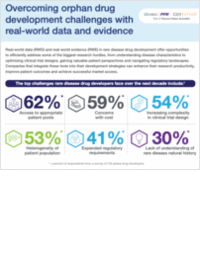
"Complex early development studies -- managing first-in-human (FIH) to Phase Ib"
Create a solid foundation for your future clinical studies with meticulously planned early phase trials
Maximizing success in early phase development requires a highly coordinated effort to anticipate potential challenges such as recruiting the right patients, engaging in top investigators and designing a trial that will build a platform for later-stage success.
In this white paper, experts explore the key steps in supporting a complex early development study starting with FIH through Phase Ib, as well as offering practical guidance to address unique challenges, regional requirements, and expedite clinical trials.
Offered Free by: PPD
See All Resources from: PPD
Thank you
This download should complete shortly. If the resource doesn't automatically download, please, click here.
Thank you
This download should complete shortly. If the resource doesn't automatically download, please, click here.
Thank you
This download should complete shortly. If the resource doesn't automatically download, please, click here.